About ivWatch
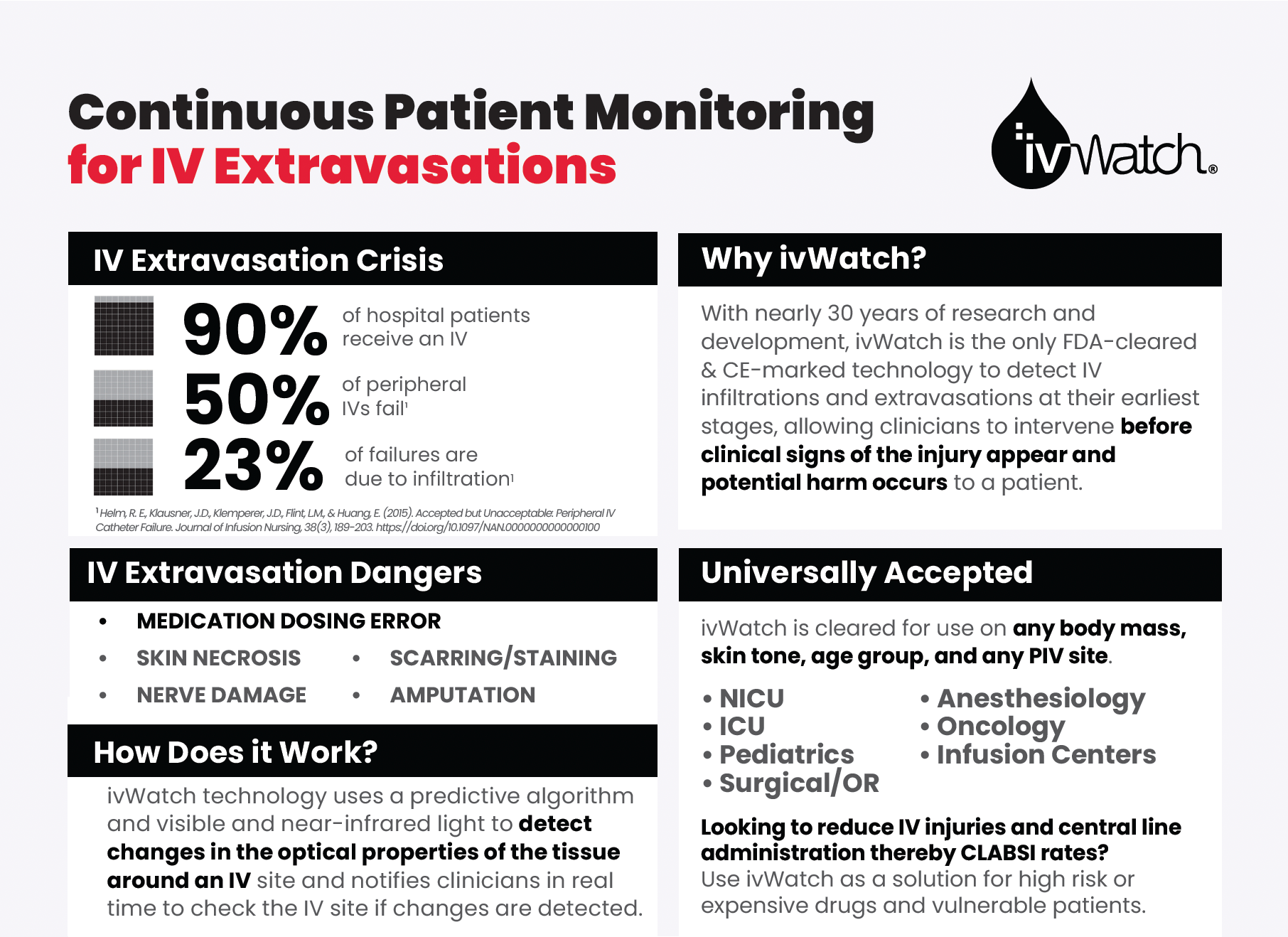
IV insertion is the most common hospital procedure, yet up to 50% of them fail, often with severe consequences ranging from dosing errors, scarring, skin staining, nerve damage, amputation, and even death. Newport News, Virginia-based ivWatch is changing that.
Founded in 2010 and holding nearly 70 patents and counting, the company has developed a first-of-its-kind Class II medical device, cleared by the FDA and CE-marked, that uses visible and near-infrared light and a predictive algorithm to continuously monitor peripheral IVs to aid in the early detection of IV leakage events, also known as infiltration and extravasation.
Applied to the skin at the IV site, ivWatch sensors perform over 18,000 checks per hour for signs of IV infiltration and extravasation, notifying healthcare workers of fluid leakage outside of the vein in real time to reduce severe adverse outcomes.
In 2024, the company surpassed more than 35 clinical proof bodies. It was also named to Fast Company’s Most Innovative Companies list, the Inc. 2024 Best in Business list in the Health Products category, and was recognized by Modern Healthcare as one of its 2024 Best in Business. The company also reached the Top 3 in the 2024 Startup World Cup Grand Finale, from an initial group of over 8,000 global startups. To learn more, visit www.ivwatch.com.